To get an accurate hydro reading, you're supposed to degas the sample. A few CO2 bubbles on the hydro can change the bouyancy and throw off your reading. Kind of hard to do that in a sight glass.Still what I don't understand is why someone hasn't developed some sort of hydrometer that is integrated into these fancy conical fermenters.

Continuous monitoring of gravity
#21

Posted 31 May 2009 - 08:31 AM
#22

Posted 31 May 2009 - 09:06 AM
Edited by JKoravos, 31 May 2009 - 09:10 AM.
#23

Posted 31 May 2009 - 09:16 AM
Believe me, I've subscribed to that method as well. But I'm a process control guy by nature, I can't escape it. I'm always thinking of ways I can improve control on my process. Generally, I don't have the time, money or inclination to implement my ideas, but I'm ramping up to do a major brewery overhaul, so these things are on my mind.Just as a point of contrast, I don't take any gravity readings whatsoever after I test for O.G. Somehow my beers still turn out great.
#24

Posted 31 May 2009 - 10:12 AM
It would be very difficult I think to mathematically determine the rate of CO2 evolution. It depends on many factors, like temperature, pressure and interfacial area between the gas & liquid. Even in the brewing literature I have that discusses ways in which to increase gas the gas exchange rate, they don't discuss how to calculate the amount of time it takes to do it (typically the reverse process but the concepts are mostly the same). As for a graph, there a was a thread on here I think recently where someone posted their gravity vs days graphs.Anyone have an idea of what the max CO2 evolution rate would be for a 10 gallon batch?For a 6.5% ABV batch you should evolve about 1000L CO2 (as associated with EtOH production). If you stretch that over a week you get an average of 94 mL/min, but obviously the majority is on the front end so the average doesn't tell you much. I need to know the evolution rate in the initial stages of fermentation to size the flow meter.Anyone have a plot of gravity versus time for a typical batch? I may be able to extract from that if the data collection rate is high enough.
#25

Posted 31 May 2009 - 10:48 AM
Well, I just need a really rough estimate for meter sizing. There are many things affecting how much CO2 is exiting the fermenter, but the main driver is fermentation rate (at least, I'm assuming it is). If I can get a good bead on that I can size the meter.It would be very difficult I think to mathematically determine the rate of CO2 evolution. It depends on many factors, like temperature, pressure and interfacial area between the gas & liquid. Even in the brewing literature I have that discusses ways in which to increase gas the gas exchange rate, they don't discuss how to calculate the amount of time it takes to do it (typically the reverse process but the concepts are mostly the same).
I saw that. I'll have to look around, I can't remember which thread it was in.As for a graph, there a was a thread on here I think recently where someone posted their gravity vs days graphs.
#26

Posted 31 May 2009 - 11:56 AM
Word, yeah, what I'm saying is that in all my reading, a lot of which has been calculuations involved in the various parts of brewing, nobody has touched that subject except to say that it's extremely variable, but there are things you can do to make it faster. I wouldn't think it' solely dependent upon the rate of fermentation, I would think that the rate of fermentation drives how much CO2 is in solution, but then you end up with unknown pressure differentials between the beer & what's in the headspace & what's outside the fermenter. I think that the best approach might be to just calculate the total CO2 that is going to come out and then figure like 90-95% of it will come out in the first 4 days, that'll give you the average rate and then maybe double that for max rate. I was going to say +85% of the rate to get the max but really I'm just making these numbers up.Well, I just need a really rough estimate for meter sizing. There are many things affecting how much CO2 is exiting the fermenter, but the main driver is fermentation rate (at least, I'm assuming it is). If I can get a good bead on that I can size the meter.
Yeah, me neither. I looked here and the other place by for some reason it didn't jump out at me.I saw that. I'll have to look around, I can't remember which thread it was in.
#27

Posted 31 May 2009 - 01:03 PM
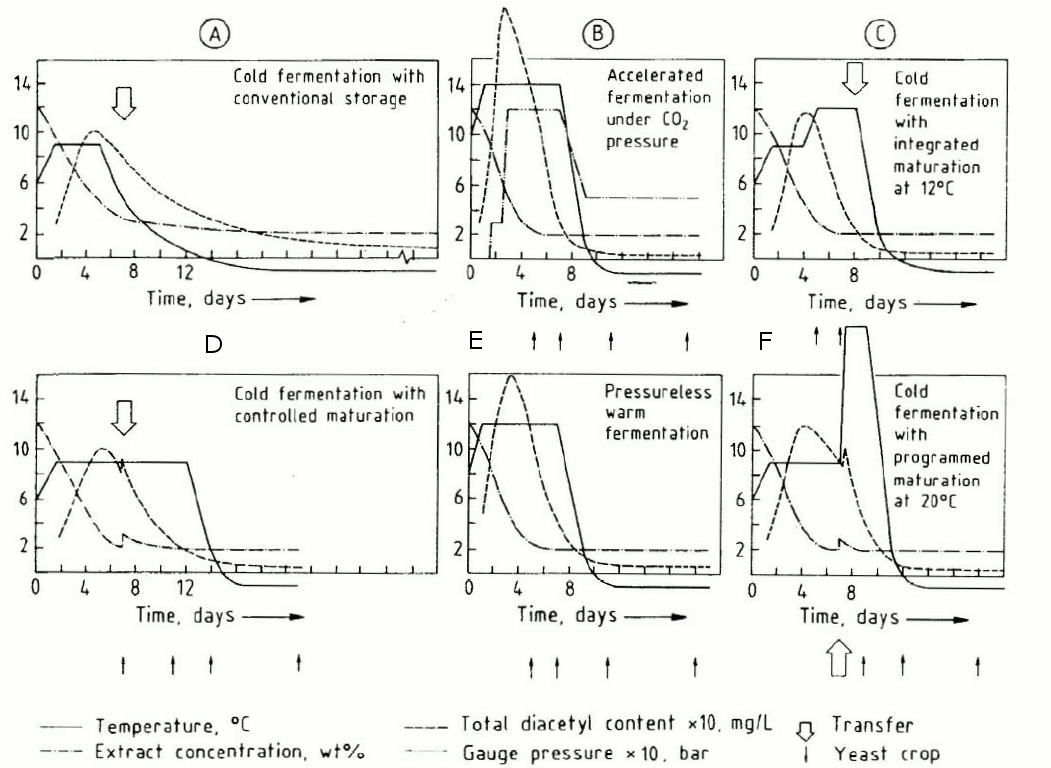
#28

Posted 31 May 2009 - 01:27 PM
The dashed line is diacetyl, I think you mean the dash-dot-dash lines which represent extract. Yeah, you might be able to back-calculate CO2 production, but not evolution from these.You might be able to back-calculate the CO2 evolution from the slope of the dashed line for the 12P beers
#29

Posted 31 May 2009 - 04:12 PM
#30

Posted 31 May 2009 - 05:02 PM
The dashed line is diacetyl, I think you mean the dash-dot-dash lines which represent extract. Yeah, you might be able to back-calculate CO2 production, but not evolution from these.
#31

Posted 31 May 2009 - 06:25 PM
#32

Posted 01 June 2009 - 07:02 PM
The amount of gas produced is precisely known. For every glucose that enters the EMP pathway you get 2 CO2 molecules.If you know your starting gravity, you know you sugar amounts and by measuring the amount of CO2 that is evolved you can precisely calculate the current gravity of your beer.It should be very straightforward if you can accurately measure the gas.BrewBasserI would think it's consistent because the there is a precise amount of carbon dioxide produced for each unit mass of sugar that is converted to alcohol. The ratio of monosaccharides to disaccharides may effect the number, I'm not sure though. I still need to do some more research. I bet Basser could shed some light on the subject.
#33

Posted 01 June 2009 - 07:18 PM
Thanks, BB.AFAYK, are there any side reactions going on that would generate enough gas to throw off the reading?The amount of gas produced is precisely known. For every glucose that enters the EMP pathway you get 2 CO2 molecules.If you know your starting gravity, you know you sugar amounts and by measuring the amount of CO2 that is evolved you can precisely calculate the current gravity of your beer.It should be very straightforward if you can accurately measure the gas.BrewBasser
#34

Posted 01 June 2009 - 07:25 PM
Not unless you have a serious bacteria infection.....BrewBasserThanks, BB.AFAYK, are there any side reactions going on that would generate enough gas to throw off the reading?
#35
 *_Guest_Matt C_*
*_Guest_Matt C_*
Posted 01 June 2009 - 07:31 PM
#36

Posted 01 June 2009 - 07:56 PM
#37

Posted 01 June 2009 - 08:16 PM
BrewBasser, isn't CO2 also released in sterol synthesis? And also in the metabolism of amino acids? People can use CO2 as a guide to how much the gravity has dropped, but I'm not certain that it is entirely from the EMP pathway. When looking at Fix's Principles in Brewing Science p. 96 I see a CO2 coming out of the transformation of mevalonic acid to squalene. Admittedly I don't know much about that reaction other than it takes energy from the yeast cells to do it, but it does look like 1 CO2 molecule is a by product. In Brewing they mention something on p. 332 about an aldehyde and a CO2 being the by-product of amino acid metabolism. Just curious what that's all about.The amount of gas produced is precisely known. For every glucose that enters the EMP pathway you get 2 CO2 molecules.If you know your starting gravity, you know you sugar amounts and by measuring the amount of CO2 that is evolved you can precisely calculate the current gravity of your beer.It should be very straightforward if you can accurately measure the gas.BrewBasser
#38

Posted 01 June 2009 - 08:18 PM
The amount of gas dissolved in the beer is very straightforward to calculate given that it is at atmospheric pressure. There are tables in almost any hombrewbook that will tell you how much gas is in the beer at a given temperature and pressure. You can safely assume that it will be saturated during the fermentation.BrewBasseri agree with what was said above. there are a lot of variables to think about like the amount of c02 that gets dissolved in to the beer even during fermentation, and also if the temp of the head space in the fermenter has any influence on the expansion of the carbon dioxide molecules too,possible false reading. I would be more concerned with a false C02 reading from some of the dissolved C02 though. does that make any sense to anyone else?

#39

Posted 03 June 2009 - 08:44 AM
Omega FMA-1714. Purchased.JK, I'm curious about the type of flow meter you were looking at, and what kind of precision you would expect. Have you made any preliminary selections?
#40

Posted 03 June 2009 - 09:06 AM
Wow, expensive toy, but you'll have fun playing with it. It looks like it operates on the same principle as a hot wire anemometer. Are you having shop calibrated for CO2 at your expected temperature and pressure, or will you apply correction factors?I will look forward to hearing about your results.Omega FMA-1714. Purchased.
1 user(s) are reading this topic
0 members, 1 guests, 0 anonymous users











