
Diacetyl rests in ales vs. lagers
#21

Posted 23 July 2010 - 05:15 AM
#22

Posted 23 July 2010 - 07:15 AM
Maybe you're not using a yeast that throws a lot of diacetyl.No offense taken.However, I'm still confused about why your theory doesn't agree with my experiments. I pitch large amounts of lager yeast into all-barley worts, let it ferment out, ramp down to lagering temperatures over a week or two, rack to secondary, and don't taste any diacetyl (I've also tried crash cooling instead of slowly ramping, and I feel like the beers are less crisp although neither method results in diacetyl).If diacetyl rests are required, then why don't I taste diacetyl in my beers without diacetyl rests?
#23

Posted 23 July 2010 - 07:58 AM
he needs to try out some 2308Maybe you're not using a yeast that throws a lot of diacetyl.
#24

Posted 23 July 2010 - 08:08 AM
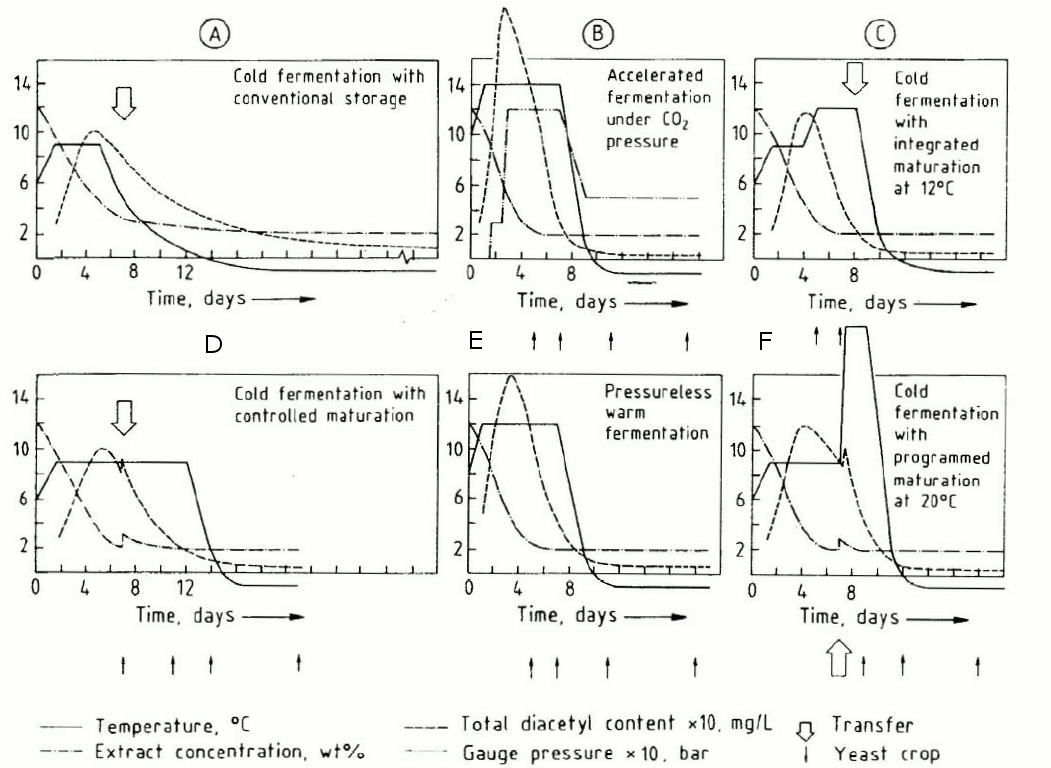
2206 is my go-to lager strain, and the Wyeast page for 2206 says "Benefits from diacetyl rest at 58°F(14°C) for 24 hours after fermentation is complete."The other question is why graphs A through E on this chart from the Technical University of Vienna are wrong. They all say that it is possible to get rid of diacetyl with a d-rest, although a d-rest (graph F) also works:Maybe you're not using a yeast that throws a lot of diacetyl.
#25

Posted 23 July 2010 - 08:30 AM
#26

Posted 23 July 2010 - 08:38 AM
from the page where I got that image, https://www.homebrew...rmenting_Lagerswhat does maturation mean in the context of those charts?
Once the primary fermentation is considered done the final gravity has not been reached yet and fermentation byproducts like diacetyl and acedealdehyde need to be reduced by the yeast. This process is called maturation of the beer and in the converional fermentation approach for lagers, as outlined above, it happens during the long cold storage.
#27

Posted 23 July 2010 - 09:38 AM
#28

Posted 23 July 2010 - 09:40 AM
I meant that sentence to say "They (referring to graphs A through E) all say that it is possible to get rid of diacetyl WITHOUT a d-rest, although a d-rest (graph F) also works."Graphs A and D both indicate that you can have a beer with no diacetyl if your fermentation and storage temperature never exceed 9C, which is 48.2F. Basser is saying that a diacetyl rest is necessary. Either the graphs from Vienna are wrong, Basser is wrong, or I have interpreted something wrong.I guess I don't see what's wrong about those graphs. Also I'm having trouble parsing this sentence: They all say that it is possible to get rid of diacetyl with a d-rest, although a d-rest (graph F) also works.
#29

Posted 23 July 2010 - 09:47 AM
#30

Posted 23 July 2010 - 10:00 AM
What do you mean by diacetyl rest? If you mean "a temperature after which there is no diacetyl" then by definition, any diacetyl-free beer will have had a diacetyl rest. If you mean, "a period of aging at elevated temperature to get rid of diacetyl", then 4 of those graphs don't have d-rests, and graph C does the d-rest at 12C or 53.6F, which is a lot lower than I was taught to do for d-rests.They all have diacetyl rests but the rest in F is at a higher temperature, which seems to remove the diacetyl more effectively. So it may not be necessary to do a high temperature d-rest if you lager long enough. It would be nice to see if you lagered long enough whether the diacetyl in A dropped as low as F. Personally, I think it might be slightly better to do the d-rest prior to lagering when the beer is still in contact with the bulk of the yeast. However, if you lagered long enough, it might be 6 of one.
#31

Posted 23 July 2010 - 10:20 AM
#32

Posted 23 July 2010 - 10:50 AM
If that's the definition, then I'll change my position from "diacetyl rests are unnecessary" to "diacetyl rests at elevated temperatures are unnecessary". Lagering is very important to brewing a lager, in my experience. Aging at elevated temperatures is NOT important, and can be detrimental if done too early.I mean a period of aging during which time diacetyl is reduced. In other words, you leave the beer on the yeast so that diacetyl can be reduced by it.
#33

Posted 23 July 2010 - 11:30 AM
BINGO! The ONLY reason temps get elevated in lagers is to make the yeast more active so it can consume the diacetyl. The temp itself has no bearing.If that's the definition, then I'll change my position from "diacetyl rests are unnecessary" to "diacetyl rests at elevated temperatures are unnecessary". Lagering is very important to brewing a lager, in my experience. Aging at elevated temperatures is NOT important, and can be detrimental if done too early.
#34

Posted 23 July 2010 - 11:36 AM
#35

Posted 23 July 2010 - 02:15 PM
A d-rest is keeping the yeast in contact with the beer so that it can reduce the vicinal diketones (VDK) of which diacetyl is the most abundant and flavorful, the other key VDK is 2,3-pentadione. You're understanding of the process of VDK production is mostly correct (see below for more info), but it does not *require* amino acid deficient wort, that only *increases* VDK production. The reaction outside the cell of converting the precurors into diacetyl and 2,3-pentadione is driven more quickly by elevating the temperature (which also increases yeast activity of course), and hence you can remove the yeast from the beer sooner if you choose to incorporate elevated temperatures in your profile.This is one reason a lot of homebrewers may not have issues with VDK in their beer if they age them for long periods of time. You can use the formula I posted in my first reply to get an estimate of how long it will take to reduce the VDK in your beer if you just lager it for a long time. You also might have a high threshold for tasting diacetyl, it's kind of hard to know exactly without doing threshold testing. One nice thing about going through sensory training with a bunch of guys and then going out and testing our senses together regularly is you realize no one person is likely to be able accurately judge all flavor compounds. There were a lot of people who would swear to me that there was no diacetyl in a beer when I picked up on it, and then as the beer warmed up the amount of diacetyl increased and they were like, oh you're right. So it's one compound I realized I have a sensitivity too, and it's probably why I don't like butter. Or maybe just not putting butter on anything for years has changed my taste such that I don't like it.But yeah, diacetyl isn't only a problem for pro brewers because of poor yeast or malt. It's problem because they are trying to bring the product to market as quickly as possible. If you're a rich enough brewery, I've heard some will use VDK levels to judge when to remove the yeast, but that kind of testing is expensive.Correction on the process of formation, about oxidation: I don't believe the oxidation of acetolactate into diacetyl requires the presence of molecular oxygen. It is an oxidative decarboxylation process in which acetolactate loses a CO2 molecule. The term oxidation really just means the loss of electrons, while reduction is gaining electrons, and there doesn't need to be oxygen involved in redox reactions. If you package a beer high in acetolactate it will overtime slowly turn into diacetyl. However, usually packaged beer is stored cold, so when you pour a beer with a lot of diacetyl precursor in it, it might taste okay at first, but as the glass warms the temperature and available oxygen speed up this process. Noticing that is one way to differentiate between technological (yeast created) and natural (bacteria created) diacetyl when you're tasting beer at a bar.Just wrote all that from memory on the train, so I hope I got everything rightWhat do you mean by diacetyl rest? If you mean "a temperature after which there is no diacetyl" then by definition, any diacetyl-free beer will have had a diacetyl rest. If you mean, "a period of aging at elevated temperature to get rid of diacetyl", then 4 of those graphs don't have d-rests, and graph C does the d-rest at 12C or 53.6F, which is a lot lower than I was taught to do for d-rests.
#36

Posted 23 July 2010 - 07:34 PM
I'm working through this thread, so this response is before seeing any after you.Couple of possibilities.1. You don't perceive diacetyl that well. Many people are actually blind to this compound and can't smell it at all.2. You ferment out longer, and or lager long enough that you get rid of it.It doesn't take months for the diacetyl to go away, and depends a bunch on your yeast. You may just be doing it right, and by your post, although your lager times are short, you just might be.Doesn't rule out the science.MolBasserNo offense taken.However, I'm still confused about why your theory doesn't agree with my experiments. I pitch large amounts of lager yeast into all-barley worts, let it ferment out, ramp down to lagering temperatures over a week or two, rack to secondary, and don't taste any diacetyl (I've also tried crash cooling instead of slowly ramping, and I feel like the beers are less crisp although neither method results in diacetyl).If diacetyl rests are required, then why don't I taste diacetyl in my beers without diacetyl rests?
#37

Posted 23 July 2010 - 07:37 PM
No this is right. How does this affect the conversation. Yeast make it, then take it up and get rid of it as fermentation ends/ after fermentation.MolBasserAnother question for Basser:My understanding of the pathway is that Yeast produce alpha acetolactate as a by-product of synthesizing amino acids leucine, isoleucine and valine, and that alpha-acetolactate is oxidized into diacetyl outside the yeast cells. During a diacetyl rest, the yeast re-absorb the diacetyl, and express enzymes to turn it into a chemical with a much lower flavor threshold.What part of that is incorrect? Do yeast produce alpha acetolactate through other pathways? This article seems to say that barley and wheat are relatively rich in leucine, isoleucine and valine.Also, this image purportedly from the University of Vienna describes six commonly used lager fermentation profiles in traditional German lager brewing, including diacetyl concentration. All six profiles get rid of diacetyl, and only one of them (the sixth, F) has a traditional diacetyl rest. In all of the others the yeast deal with diacetyl at fermentation temperatures. Is this incorrect? Why don't yeast deal with diacetyl at fermentation temperatures?
#38

Posted 23 July 2010 - 07:40 PM
Yes. This is true. You just need to give the time for the yeast to get rid of it.This was the source of our misscommunication. My bad.(although heating it up will accelerate the process)MolBasserIf that's the definition, then I'll change my position from "diacetyl rests are unnecessary" to "diacetyl rests at elevated temperatures are unnecessary". Lagering is very important to brewing a lager, in my experience. Aging at elevated temperatures is NOT important, and can be detrimental if done too early.
Edited by MolBasser, 23 July 2010 - 07:40 PM.
#39

Posted 23 July 2010 - 08:23 PM
#40
 *_Guest_Matt C_*
*_Guest_Matt C_*
Posted 23 July 2010 - 08:47 PM
Had a BIG time butter bomb. Fermented US-05 at near lager temps,as far as dry yeast goes that yeast worked great at lager temps,however,I forgot to raise the temps back up to the recommended temps and instead went to keg with and served there after.Has anyone actually had a problem in homebrew with too much diacetyl? My experience is that when I do a D-rest too early my lagers taste a litte "ale-like", and when I skip the D-rest I never have any problems.
1 user(s) are reading this topic
0 members, 1 guests, 0 anonymous users












